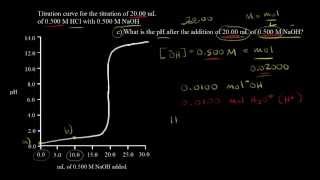
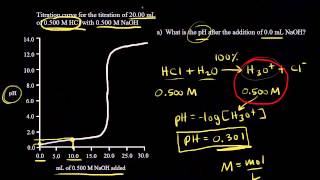
18. Aqueous Equilibrium
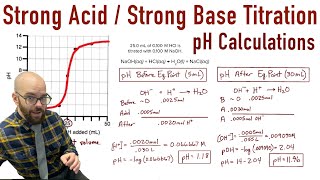
Titrations: Strong Acid-Strong Base

Get help from an AI Tutor
Ask a question to get started.
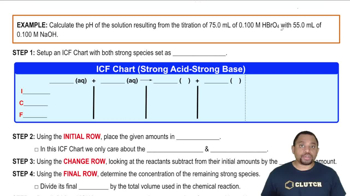
Problem 131
Textbook Question
Textbook QuestionPeople often take milk of magnesia to reduce the discomfort associated with acid stomach or heartburn. The recommended dose is 1 teaspoon, which contains 4.00 * 102 mg of Mg(OH)2. What volume of an HCl solution with a pH of 1.3 can be neutralized by one dose of milk of magnesia? If the stomach contains 2.00 * 102 mL of pH 1.3 solution, is all the acid neutralized? If not, what fraction is neutralized?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
980
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos