13. Liquids, Solids & Intermolecular Forces
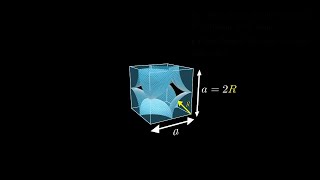
Simple Cubic Unit Cell

Get help from an AI Tutor
Ask a question to get started.
Problem 138
Textbook Question
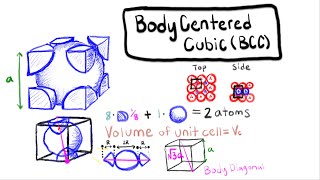
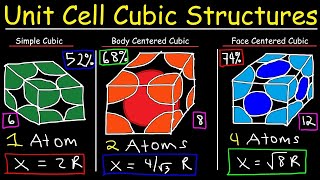
Textbook QuestionAssume that 1.588 g of an alkali metal undergoes complete reaction with the amount of gaseous halogen contained in a 0.500 L flask at 298 K and 755 mm Hg pressure. In the reaction, 22.83 kJ is released 1ΔH = -22.83 kJ2. The product, a binary ionic compound, crystallizes in a unit cell with anions in a face-centered cubic arrangement and with cations centered along each edge between anions. In addition, there is a cation in the center of the cube. (c) Sketch a space-filling, head-on view of the unit cell, labeling the ions. Are the anions in contact with one another?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
14mPlay a video:
268
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 6 videos