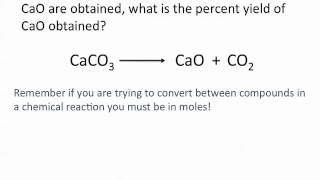
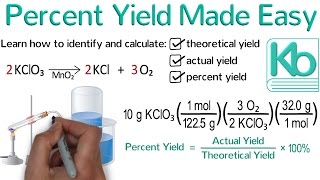
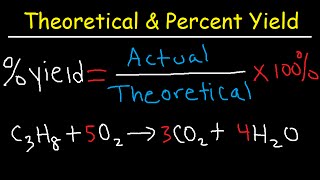
3. Chemical Reactions
Percent Yield

Get help from an AI Tutor
Ask a question to get started.
Problem 8
Textbook Question
Textbook QuestionNitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO and O2 shown in the accompanying diagram. The blue spheres represent N, and the red ones represent O. (c) If the actual yield of the reaction was 75% instead of 100%, how many molecules of each kind would be present after the reaction was over?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
666
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos