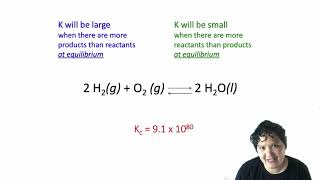16. Chemical Equilibrium
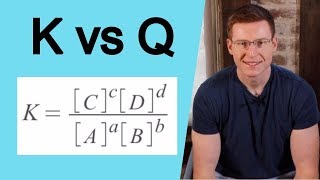
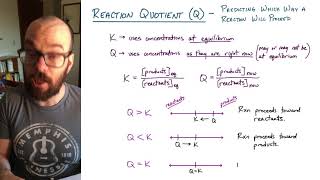
Reaction Quotient

Get help from an AI Tutor
Ask a question to get started.
Problem 118
Textbook Question
Textbook QuestionConsider the following equilibrium: Ag + 1aq2 + Cl-1aq2 ∆ AgCl1s2 Use Le Châtelier's principle to predict how the amount of solid silver chloride will change when the equilibrium is disturbed by: (d) Removing Cl-; also account for the change using the reaction quotient Qc
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
205
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos