18. Aqueous Equilibrium
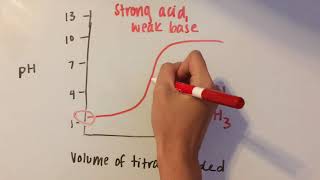
Intro to Acid-Base Titration Curves

Get help from an AI Tutor
Ask a question to get started.
Problem 90b
Textbook Question
Textbook QuestionA 1.248-g sample of limestone rock is pulverized and then treated with 30.00 mL of 1.035 M HCl solution. The excess acid then requires 11.56 mL of 1.010 M NaOH for neutralization. Calculate the percentage by mass of calcium carbonate in the rock, assuming that it is the only substance reacting with the HCl solution.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
800
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos