3. Chemical Reactions
Percent Yield

Get help from an AI Tutor
Ask a question to get started.
Problem 50
Textbook Question
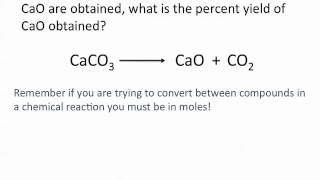
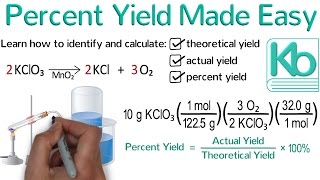
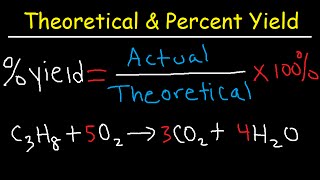
Textbook QuestionMagnesium oxide can be made by heating magnesium metal in the presence of oxygen. The balanced equation for the reaction is: 2 Mg(s) + O2( g)¡2 MgO(s) When 10.1 g of Mg reacts with 10.5 g O2, 11.9 g MgO is collected. Determine the limiting reactant. Determine the theoretical yield. Determine the percent yield for the reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
8843
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos