11. Bonding & Molecular Structure
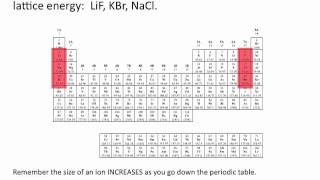
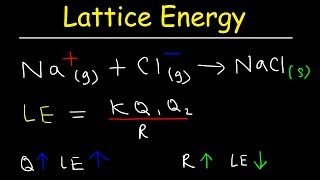
Lattice Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 2
Textbook Question
Textbook QuestionIllustrated are four ions — A, B, X, and Y— showing their relative ionic radii. The ions shown in red carry positive charges: a 2+ charge for A and a 1+ charge for B. Ions shown in blue carry negative charges: a 1- charge for X and a 2- charge for Y. (b) Among the combinations in part (a), which leads to the ionic compound having the largest lattice energy?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
417
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos