11. Bonding & Molecular Structure
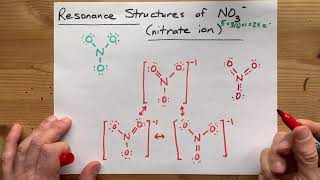
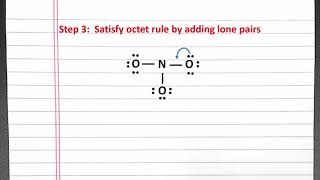
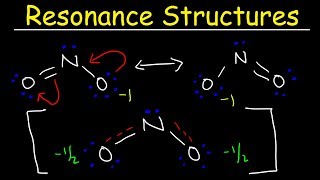
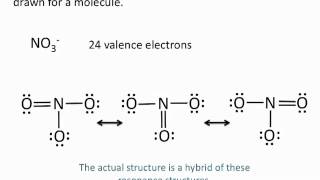
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 52
Textbook Question
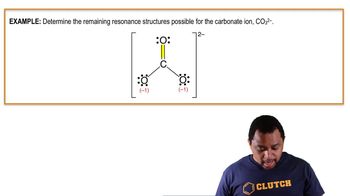
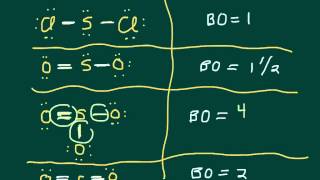
Textbook QuestionFor each of the following molecules or ions of sulfur and oxygen, write a single Lewis structure that obeys the octet rule, and calculate the oxidation numbers and formal charges on all the atoms: (c) SO32- Write a single Lewis structure that obeys the octet rule for SO3 2 - and assign the formal charges on all the atoms.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
379
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos