16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 89
Textbook Question
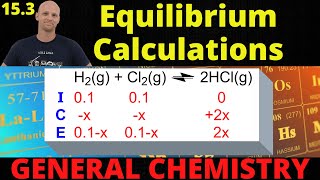
Textbook QuestionAt 700 K, the equilibrium constant for the reaction CCl41g2 Δ C1s2 + 2 Cl21g2 is Kp = 0.76. A flask is charged with 2.00 atm of CCl4, which then reaches equilibrium at 700 K. (b) What are the partial pressures of CCl4 and Cl2 at equilibrium?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
11mPlay a video:
1030
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos