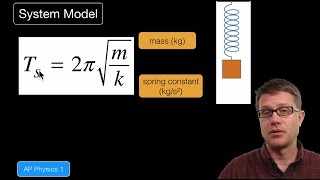
8. Thermochemistry
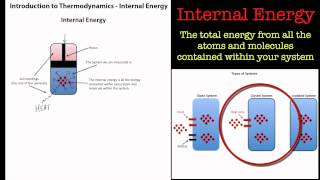
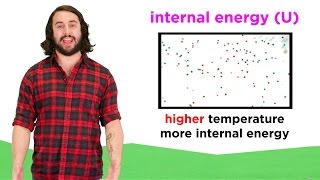
Internal Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 111
Textbook Question
Textbook QuestionA 20.0-L volume of an ideal gas in a cylinder with a piston is at a pressure of 3.0 atm. Enough weight is suddenly removed from the piston to lower the external pressure to 1.5 atm. The gas then expands at constant temperature until its pressure is 1.5 atm. Find w.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1926
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos