15. Chemical Kinetics
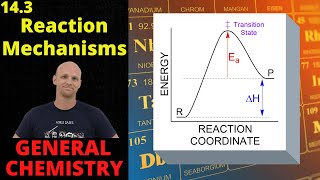
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 109a
Textbook Question
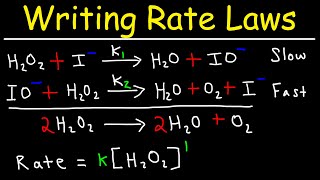
Textbook QuestionThe gas-phase decomposition of ozone is thought to occur by the following two-step mechanism. Step 1: O31g2ΔO21g2 + O1g2 (fast) Step 2: O1g2 + O31g2¡2 O21g2 (slow) (d) If instead the reaction occurred in a single step, would the rate law change? If so, what would it be?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
838
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos