15. Chemical Kinetics
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 89
Textbook Question
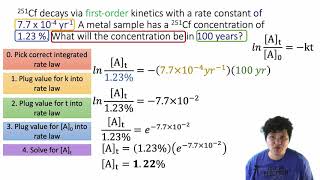
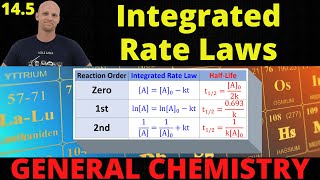
Textbook QuestionDinitrogen pentoxide decomposes in the gas phase to form nitrogen dioxide and oxygen gas. The reaction is first order in dinitrogen pentoxide and has a half-life of 2.81 h at 25 °C. If a 1.5-L reaction vessel initially contains 745 torr of N2O5 at 25 °C, what partial pressure of O2 is present in the vessel after 215 minutes?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
4295
views
1
rank
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos