3. Chemical Reactions
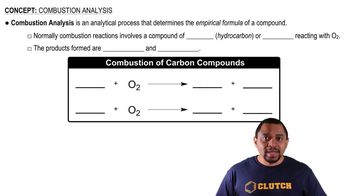
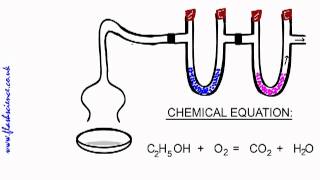
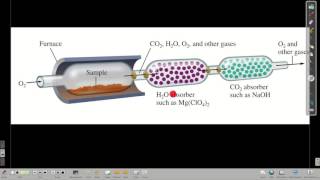
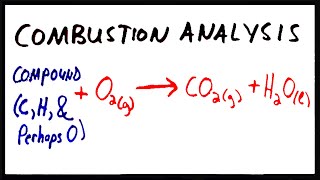
Combustion Analysis

Get help from an AI Tutor
Ask a question to get started.
Problem 111
Textbook Question
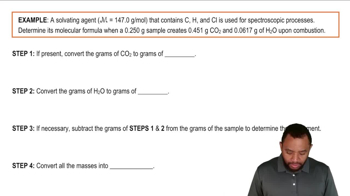
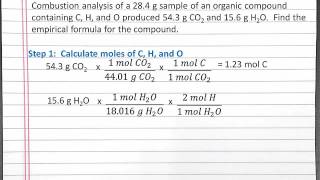
Textbook QuestionCompound X contains only carbon, hydrogen, nitrogen, and chlorine. When 1.00 g of X is dissolved in water and allowed to react with excess silver nitrate, AgNO3, all the chlorine in X reacts and 1.95 g of solid AgCl is formed. When 1.00 g of X undergoes complete combustion, 0.900 g of CO2 and 0.735 g of H2O are formed. What is the empirical formula of X?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1034
views
1
rank
2
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos