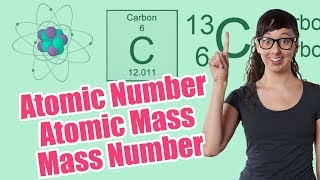2. Atoms & Elements
Atomic Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 75
Textbook Question
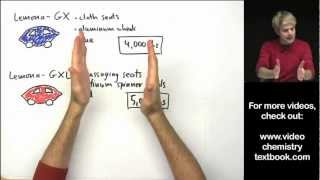
Textbook QuestionAn element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu. Find the atomic mass of this element and identify it.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
8288
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos