14. Solutions
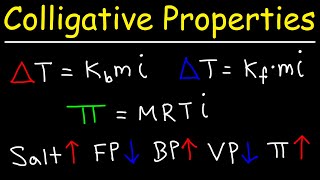
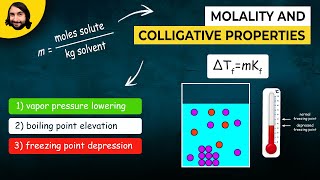
Boiling Point Elevation

Get help from an AI Tutor
Ask a question to get started.
Problem 116
Textbook Question
Textbook QuestionA solution of citric acid, C6H8O7, in 50.0 g of acetic acid has a boiling point elevation of ΔT = 1.76 °C. What is the molality of the solution if the molal boilin# g-point-elevation constant for acetic acid is Kb = 3.07 1°C kg2>mol.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
530
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos