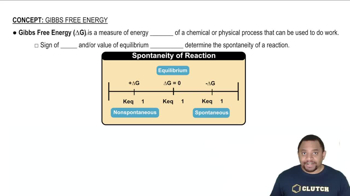
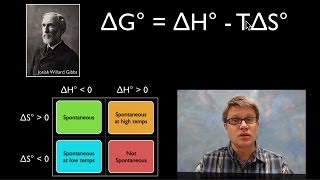
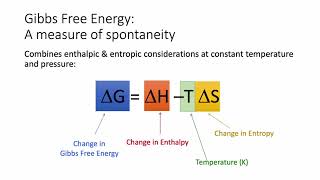
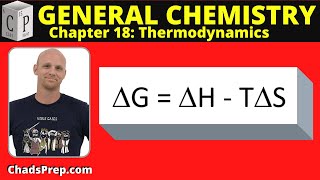
19. Chemical Thermodynamics
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 96b
Textbook Question
Textbook QuestionThe values of ΔGf° for the hydrogen halides become less negative with increasing atomic number. The ΔGf° of HI is slightly positive. However, the trend in ΔSf° is to become more positive with increasing atomic number. Explain.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
681
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos