12. Molecular Shapes & Valence Bond Theory
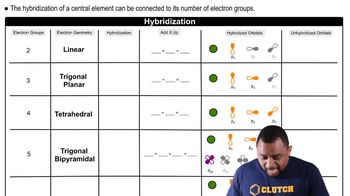
Hybridization

Get help from an AI Tutor
Ask a question to get started.
Problem 97a
Textbook Question
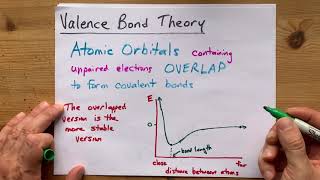
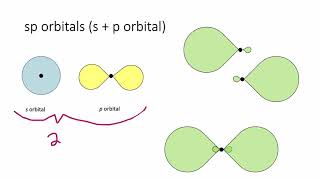
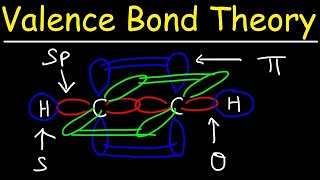
Textbook QuestionWhich of the following statements about hybrid orbitals is or are true? (i) After an atom undergoes sp hybridization, there is one unhybridized p orbital on the atom, (ii) Under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle, and (iii) The angle between the large lobes of sp3 hybrids is 109.5°.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
38sPlay a video:
1592
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos