10. Periodic Properties of the Elements
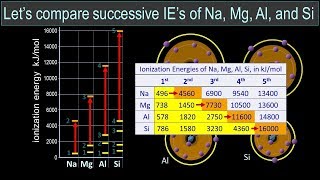
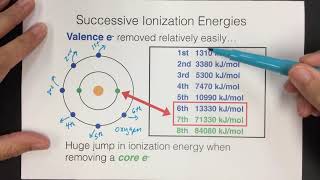
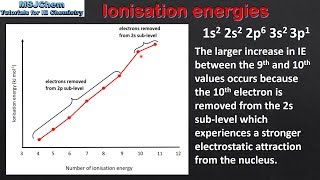
Periodic Trend: Successive Ionization Energies

Get help from an AI Tutor
Ask a question to get started.
Problem 39
Textbook Question
Textbook QuestionWhich element has the highest second ionization energy: Li, K, or Be?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
528
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos