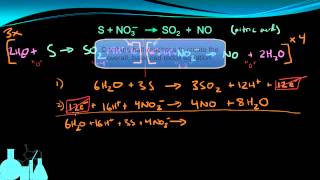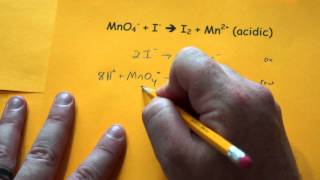6. Chemical Quantities & Aqueous Reactions
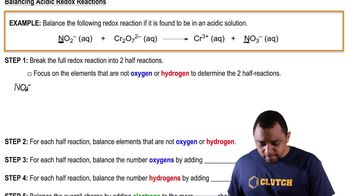
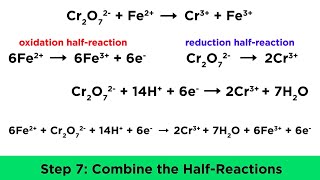
Balancing Redox Reactions: Acidic Solutions

Get help from an AI Tutor
Ask a question to get started.
Problem 97
Textbook Question
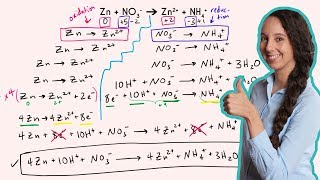
Textbook QuestionA disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions: (b) MnO42-1aq2 ¡ MnO4-1aq2 + MnO21s2 (acidic solution)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
966
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos