11. Bonding & Molecular Structure
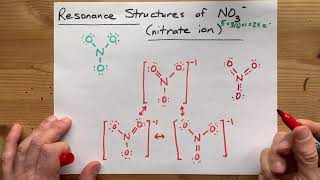
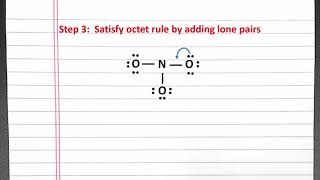
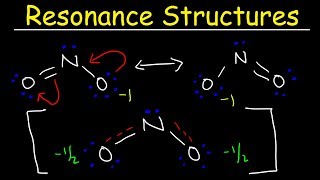
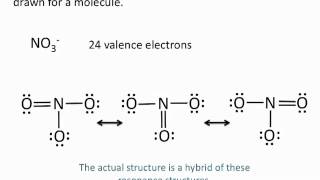
Resonance Structures

Get help from an AI Tutor
Ask a question to get started.
Problem 88
Textbook Question
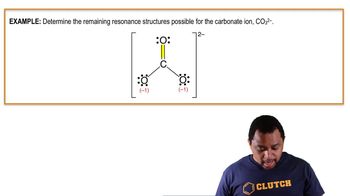
Textbook QuestionA major challenge in implementing the 'hydrogen economy' is finding a safe, lightweight, and compact way of storing hydrogen for use as a fuel. The hydrides of light metals are attractive for hydrogen storage because they can store a high weight percentage of hydrogen in a small volume. For example, NaAlH4 can release 5.6% of its mass as H2 upon decomposing to NaH1s2, Al1s2, and H21g2. NaAlH4 possesses both covalent bonds, which hold polyatomic anions together, and ionic bonds. (d) What is the formal charge on hydrogen in the polyatomic ion?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
199
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos