8. Thermochemistry
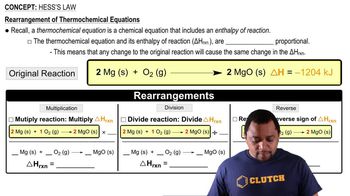
Hess's Law

Get help from an AI Tutor
Ask a question to get started.
Problem 64b
Textbook Question
Textbook QuestionFrom the enthalpies of reaction 2 C1s2 + O21g2¡ 2 CO1g2 H = -221.0 kJ 2 C1s2 + O21g2 + 4 H21g2¡ 2 CH3OH1g2 H = -402.4 kJ calculate H for the reaction CO1g2 + 2 H21g2¡CH3OH1g2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
284
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos