13. Liquids, Solids & Intermolecular Forces
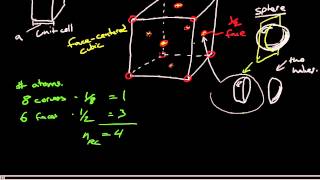
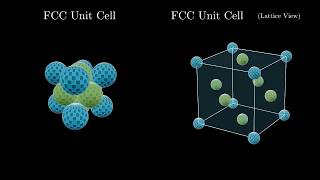
Face Centered Cubic Unit Cell

Get help from an AI Tutor
Ask a question to get started.
Problem 142
Textbook Question
Textbook QuestionThe alkali metal fulleride superconductors M3C60 have a cubic closest-packed (face-centered cubic) arrangement of nearly spherical C60 3- anions with M+ cations in the holes between the larger C603- ions. The holes are of two types: octahedral holes, which are surrounded octahedrally by six C603- ions; and tetrahedral holes, which are surrounded tetrahedrally by four C603- ions. (c) Specify fractional coordinates for all the octahedral and tetrahedral holes. (Fractional coordinates are fractions of the unit cell edge lengths. For example, a hole at the center of the cell has fractional coordinates 12, 12, 12.)

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
302
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos