17. Acid and Base Equilibrium
Ka and Kb

Get help from an AI Tutor
Ask a question to get started.
Problem 101
Textbook Question
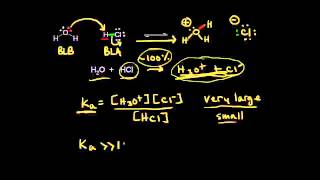
Textbook QuestionSalts containing the phosphate ion are added to municipal water supplies to prevent the corrosion of lead pipes. (a) Based on the pKa values for phosphoric acid 1pKa1 = 7.5 * 10 - 3, pKa2 = 6.2 * 10 - 8, pKa3 = 4.2 * 10 - 132 what is the Kb value for the PO43 - ion?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
247
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos