15. Chemical Kinetics
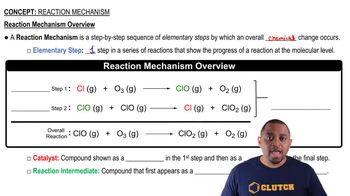
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 106b
Textbook Question
Textbook QuestionConsider the reaction: 2 NH3(aq) + OCl - (aq)¡N2H4(aq) + H2O(l ) + Cl - (aq) This three-step mechanism is proposed: NH3(aq) + OCl - (aq) Δk1k2NH2Cl(aq) + OH- (aq) Fast NH2Cl(aq) + NH3(aq) ¡k3N2H5+ (aq) + Cl - (aq) Slow N2H5+ (aq) + OH- (aq) ¡k4N2H4(aq) + H2O(l ) Fast a. Show that the mechanism sums to the overall reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
676
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos