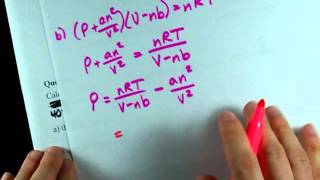7. Gases
Van der Waals Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 92
Textbook Question
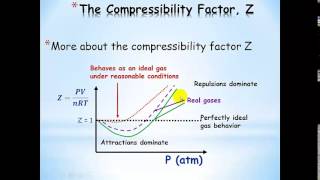
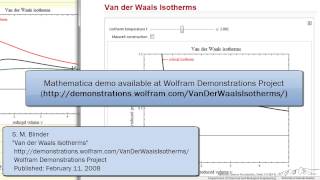
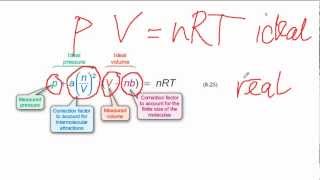
Textbook QuestionBased on their respective van der Waals constants ( Table 10.3), is Ar or CO2 expected to behave more nearly like an ideal gas at high pressures?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
756
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos