12. Molecular Shapes & Valence Bond Theory
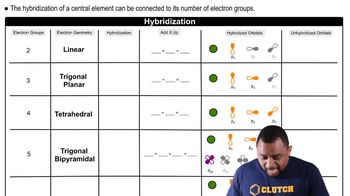
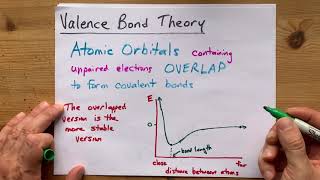
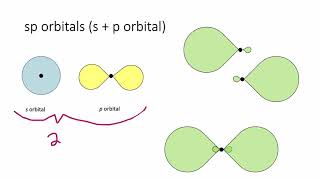
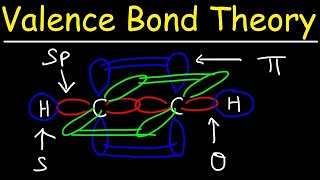
Hybridization

Get help from an AI Tutor
Ask a question to get started.
Problem 72
Textbook Question
Textbook QuestionAspirin has the following connections among atoms. Complete the electron-dot structure for aspirin, tell how many s bonds and how many p bonds the molecule contains, and tell the hybridization of each carbon atom.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
490
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos