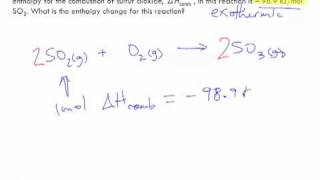8. Thermochemistry
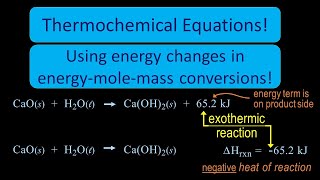
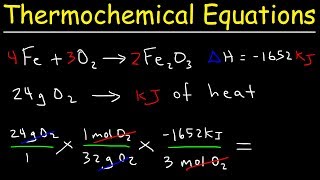
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 98
Textbook Question
Textbook QuestionThe automobile fuel called E85 consists of 85% ethanol and 15% gasoline. E85 can be used in the so-called flex-fuel vehicles (FFVs), which can use gasoline, ethanol, or a mix as fuels. Assume that gasoline consists of a mixture of octanes (different isomers of C8H18), that the average heat of combustion of C8H181l2 is 5400 kJ>mol, and that gasoline has an average density of 0.70 g>mL. The density of ethanol is 0.79 g>mL. (a) By using the information given as well as data in Appendix C, compare the energy produced by combustion of 1.0 L of gasoline and of 1.0 L of ethanol.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
1545
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos