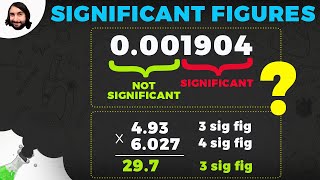
1. Intro to General Chemistry
Significant Figures

Get help from an AI Tutor
Ask a question to get started.
Problem 69
Textbook Question
Textbook QuestionTwo students determine the percentage of lead in a sample as a laboratory exercise. The true percentage is 22.52%. The students' results for three determinations are as follows: (1) 22.52, 22.48, 22.54 (2) 22.64, 22.58, 22.62 (b) Precision can be judged by examining the average of the deviations from the average value for that data set. (Calculate the average value for each data set; then calculate the average value of the absolute deviations of each measurement from the average.) Which set is more precise?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
209
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos