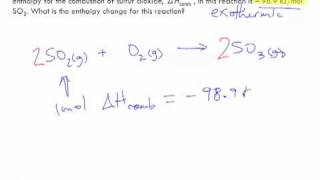8. Thermochemistry
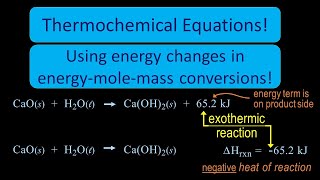
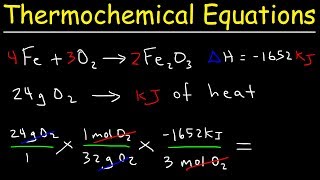
Thermochemical Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 91
Textbook Question
Textbook QuestionAssume that 100.0 mL of 0.200 M CsOH and 50.0 mL of 0.400 M HCl are mixed in a calorimeter. The solutions start out at 22.50 °C, and the final temperature after reaction is 24.28 °C. The densities of the solutions are all 1.00 g/mL, and the specific heat of the mixture is 4.2 J/(g•°C). What is the enthalpy change for the neutralization reaction of 1.00 mol of CsOH in kJ?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
413
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos