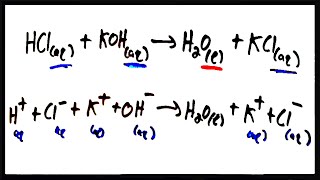6. Chemical Quantities & Aqueous Reactions
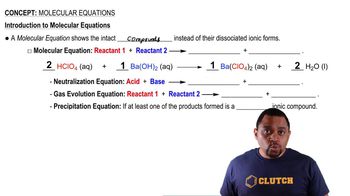
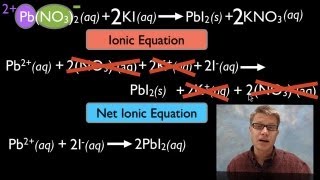
Molecular Equations

Get help from an AI Tutor
Ask a question to get started.
Problem 122a
Textbook Question
Textbook QuestionA solution contains one or more of the following ions: Hg2 2 + , Ba2 + , and Fe2 + . When you add potassium chloride to the solution, a precipitate forms. The precipitate is filtered off, and you add potassium sulfate to the remaining solution, producing no precipitate. When you add potassium carbonate to the remaining solution, a precipitate forms. Which ions were present in the original solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1179
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos