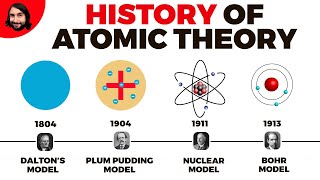
2. Atoms & Elements
Atomic Theory

Get help from an AI Tutor
Ask a question to get started.
Problem 11
Textbook Question
Textbook QuestionA 1.0-g sample of carbon dioxide (CO2) is fully decomposed into its elements, yielding 0.273 g of carbon and 0.727 g of oxygen. If a sample of a different compound decomposes into 0.429 g of carbon and 0.571 g of oxygen, what is its ratio of the mass of O to C? (c) According to Dalton's atomic theory, what is the empirical formula of the second compound?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1467
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos