14. Solutions
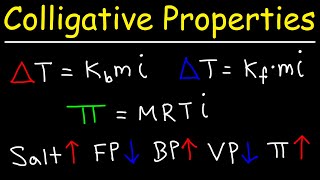
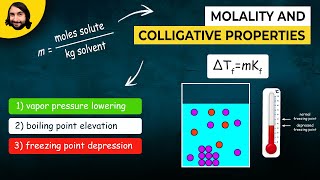
Freezing Point Depression

Get help from an AI Tutor
Ask a question to get started.
Problem 114
Textbook Question
Textbook QuestionWhen HNO2 is dissolved in water, it partially dissociates according to the equation HNO2ΔH+ + NO2- . A solution is prepared that contains 7.050 g of HNO2 in 1.000 kg of water. Its freezing point is -0.2929 °C. Calculate the fraction of HNO2 that has dissociated.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2539
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos