7. Gases
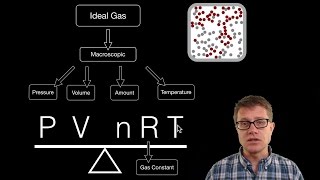
The Ideal Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 42a
Textbook Question
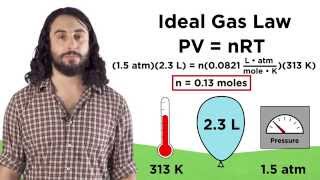
Textbook QuestionA 334-mL cylinder for use in chemistry lectures contains 5.225 g of helium at 23 °C. How many grams of helium must be released to reduce the pressure to 7.60 MPa assuming ideal gas behavior?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
572
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos