16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 162
Textbook Question
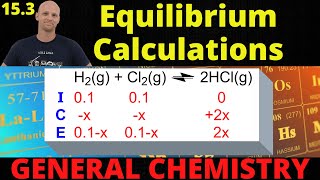
Textbook QuestionFor the decomposition reaction PCl51g2 ∆ PCl31g2 + Cl21g2, Kp = 381 at 600 K and Kc = 46.9 at 700 K. (b) If 1.25 g of PCl5 is introduced into an evacuated 0.500-L flask at 700 K and the decomposition reaction is allowed to reach equilibrium, what percent of the PCl5 will decompose and what will be the total pressure in the flask?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
321
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos