19. Chemical Thermodynamics
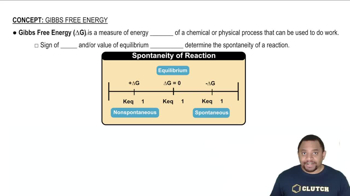
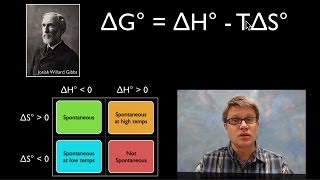
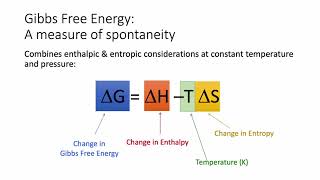
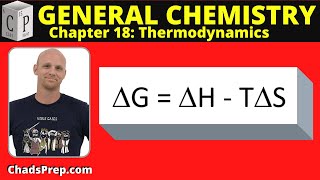
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 128b
Textbook Question
Textbook QuestionThe normal boiling point of bromine is 58.8 °C, and the standard entropies of the liquid and vapor are S°[Br2(l) = 152.2 J/(K*mol); S°[Br2(g) = 245.4 J/(K*mol). At what temperature does bromine have a vapor pressure of 227 mmHg?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1418
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos