16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 80
Textbook Question
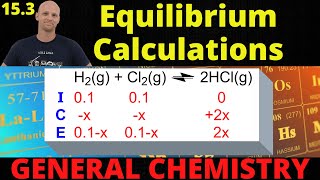
Textbook QuestionFor the equilibrium PH3BCl31s2 Δ PH31g2 + BCl31g2 Kp = 0.052 at 60 C. (b) After 3.00 g of solid PH3BCl3 is added to a closed 1.500-L vessel at 60 C, the vessel is charged with 0.0500 g of BCl31g2. What is the equilibrium concentration of PH3?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
941
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos