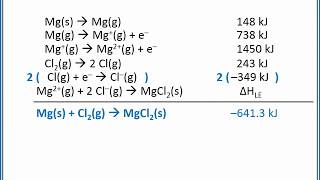11. Bonding & Molecular Structure
Born Haber Cycle

Get help from an AI Tutor
Ask a question to get started.
Problem 96
Textbook Question
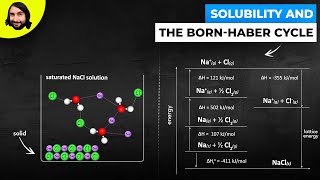
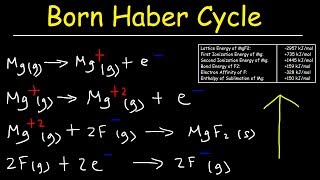
Textbook QuestionUse the following information plus the data given in Tables 6.2 and 6.3 to calculate the second electron affinity, Eea2, of oxygen. Is the O2-ion stable in the gas phase? Why is it stable in solid MgO? Heat of sublimation for Mg1s2 = +147.7 kJ/mol Bond dissociation energy for O21g2 = +498.4 kJ/mol Eea1 for O1g2 = -141.0 kJ/mol Net energy change for formation of MgO(s) from its elements = -601.7 kJ/mol
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
324
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos