3. Chemical Reactions
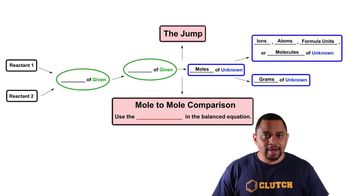
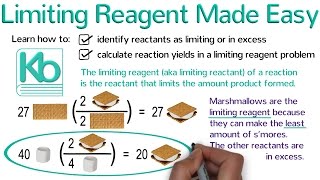
Limiting Reagent

Get help from an AI Tutor
Ask a question to get started.
Problem 40
Textbook Question
Textbook QuestionConsider the reaction: 2 CH3OH(g) + 3 O2( g)¡2 CO2( g) + 4 H2O(g) Each of the molecular diagrams represents an initial mixture of the reactants. How many CO2 molecules form from the reaction mixture that produces the greatest amount of products?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
10mPlay a video:
2035
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos