8. Thermochemistry
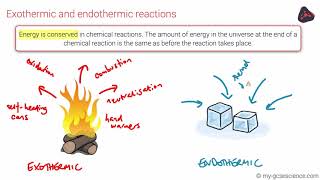
Endothermic & Exothermic Reactions

Get help from an AI Tutor
Ask a question to get started.
Problem 26
Textbook Question
Textbook QuestionFor the following processes, calculate the change in internal energy of the system and determine whether the process is endothermic or exothermic: (a) A balloon is cooled by removing 0.655 kJ of heat. It shrinks on cooling, and the atmosphere does 382 J of work on the balloon.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
747
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos