15. Chemical Kinetics
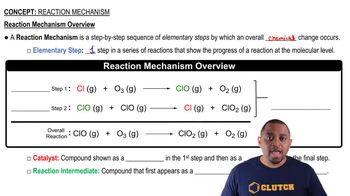
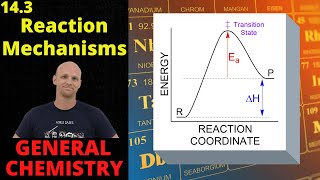
Reaction Mechanism

Get help from an AI Tutor
Ask a question to get started.
Problem 74b
Textbook Question
Textbook QuestionYou have studied the gas-phase oxidation of HBr by O2: 4 HBr1g2 + O21g2¡2 H2O1g2 + 2 Br21g2 You find the reaction to be first order with respect to HBr and first order with respect to O2. You propose the following mechanism: HBr1g2 + O21g2¡ HOOBr1g2 HOOBr1g2 + HBr1g2¡2 HOBr1g2 HOBr1g2 + HBr1g2¡ H2O1g2 + Br21g2 (b) Based on the experimentally determined rate law, which step is rate determining?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
829
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos