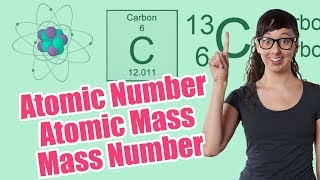2. Atoms & Elements
Atomic Mass

Get help from an AI Tutor
Ask a question to get started.
Problem 118
Textbook Question
Textbook QuestionNaturally occurring boron consists of two isotopes: 10^B (19.9%) with an isotopic mass of 10.0129 and 11^B (80.1%) with an isotopic mass of 11.009 31. What is the atomic weight of boron? Check your answer by looking at a periodic table.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1371
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos