7. Energy, Rate and Equilibrium
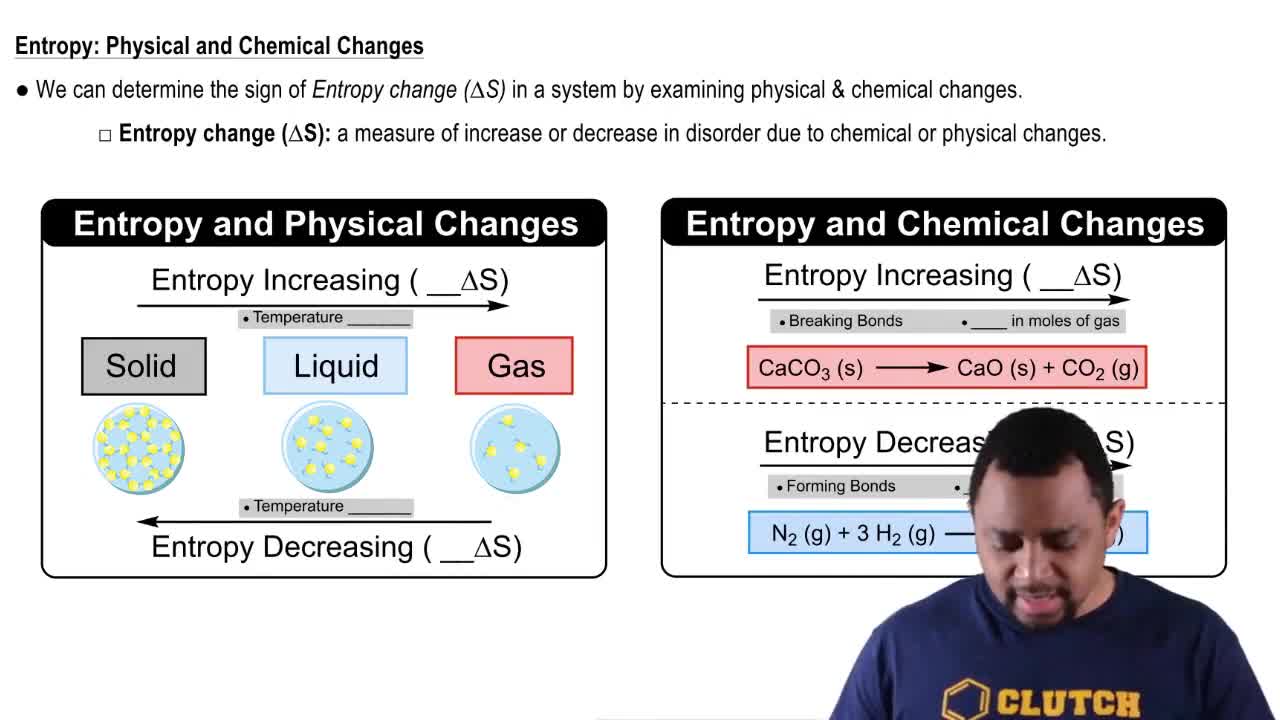
Entropy (Simplified)
Problem 37
Textbook Question
Textbook QuestionFor the reaction NaCl(s) →^(water) Na +(aq) + Cl -(aq), ∆H = +1 kcal/mol (+4.184 kJ/mol) Does entropy increase or decrease in this process?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
94
views
Was this helpful?
Related Videos
Related Practice