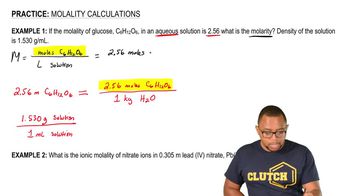9. Solutions
Molality
Problem 95
Textbook Question
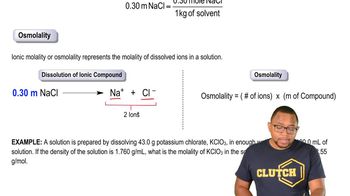
Textbook QuestionMany compounds are only partially dissociated into ions in aqueous solution. Trichloroacetic acid (CCl₃CO₂H), for instance, is partially dissociated in water according to the equation CCl₃CO₂H (aq) → H⁺ (aq) + CCl₃CO₂⁻ (aq) For a solution prepared by dissolving 1.00 mol of trichloroacetic acid in 1.00 kg of water, 36.0% of the trichloroacetic acid dissociates to form H⁺ and CCl₃CO₂⁻ ions. What is the total concentration of dissolved ions and molecules in 1 kg of water?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
176
views
Was this helpful?
Related Videos
Related Practice