6. Thermodynamics and Kinetics
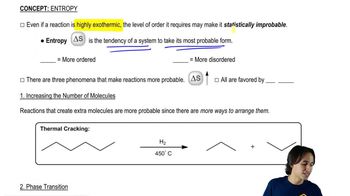
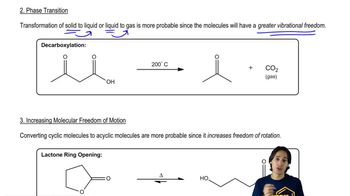
Entropy
Problem 7b
Textbook Question
Textbook QuestionThe dehydrogenation of butane to trans-but-2-ene has ΔH° = +116 kJ/mol (+27.6 kcal/mol) and ΔS° = +117J/kelvin-mol (+28.0 cal/kelvin-mol). a. Compute the value of ΔG° for dehydrogenation at room temperature (25 °C or 298 °K). Is dehydrogenation favored or disfavored? HINT: When you are doing synthesis problems, avoid using these high-temperature industrial methods. They require specialized equipment, and they produce variable mixtures of products.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
190
views
Was this helpful?
Related Videos
Related Practice