1. A Review of General Chemistry
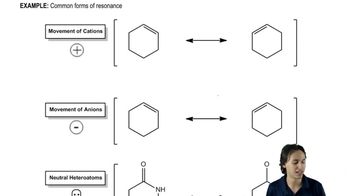
Resonance Structures
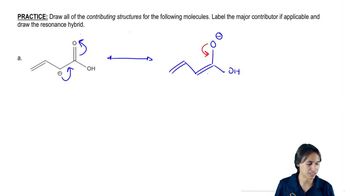
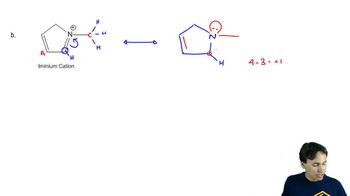
Problem 20
Textbook Question
Textbook QuestionA carboxylic acid has two oxygen atoms, each with two nonbonding pairs of electrons. (a) Draw the resonance forms of a carboxylic acid that is protonated on the hydroxy oxygen atom. (b) Compare the resonance forms with those given previously for an acid protonated on the carbonyl oxygen atom. (c) Explain why the carbonyl oxygen atom of a carboxylic acid is more basic than the hydroxy oxygen.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
555
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos