20. Heat and Temperature
Specific Heat & Temperature Changes
Multiple Choice
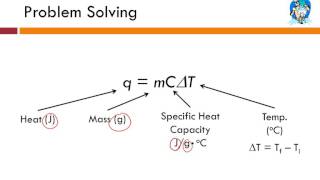
Multiple ChoiceYou are given a sample of an unknown metal. You weigh the sample and find that its weight is 29.4N. You add 1.25×104 J of heat energy to the sample and find that its temperature increases from 52°C to 70°C. What is the specific heat of this unknown metal?
A
23.6 J/(kg⋅K)
B
231.5 J/(kg⋅K)
C
14.3 J/(kg⋅K)
D
1.20×104 J/(kg⋅K)
360
views
3
rank
Related Videos
Related Practice
Showing 1 of 9 videos