21. Kinetic Theory of Ideal Gases
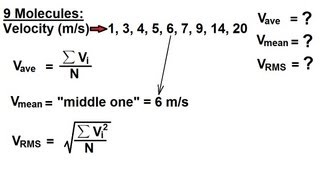
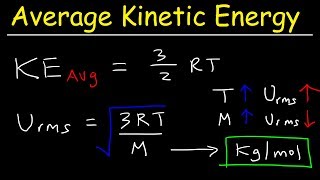
Root-Mean-Square Velocity of Gases
Problem 18d
Textbook Question
Textbook QuestionSmoke particles in the air typically have masses of the order of 10-16 kg. The Brownian motion (rapid, irregular movement) of these particles, resulting from collisions with air molecules, can be observed with a microscope. (a) Find the rootmean-square speed of Brownian motion for a particle with a mass of 3.00 * 10-16 kg in air at 300 K.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
880
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos