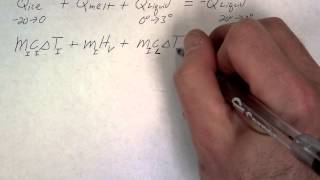20. Heat and Temperature
Calorimetry with Temperature and Phase Changes
Problem 17d
Textbook Question
Textbook QuestionA laboratory technician drops a 0.0850-kg sample of unknown solid material, at 100.0°C, into a calorimeter. The calorimeter can, initially at 19.0°C, is made of 0.150 kg of copper and contains 0.200 kg of water. The final temperature of the calorimeter can and contents is 26.1°C. Compute the specific heat of the sample
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
11mPlay a video:
307
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos