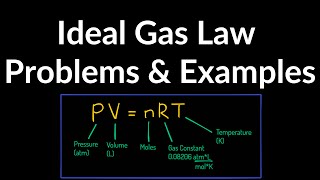
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18g
Textbook Question
Textbook QuestionMeteorology. The vapor pressure is the pressure of the vapor phase of a substance when it is in equilibrium with the solid or liquid phase of the substance. The relative humidity is the partial pressure of water vapor in the air divided by the vapor pressure of water at that same temperature, expressed as a percentage. The air is saturated when the humidity is 100%. (a) The vapor pressure of water at 20.0°C is 2.34 * 10^3 Pa. If the air temperature is 20.0°C and the relative humidity is 60%, what is the partial pressure of water vapor in the atmosphere (that is, the pressure due to water vapor alone)?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
793
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos