21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18k
Textbook Question
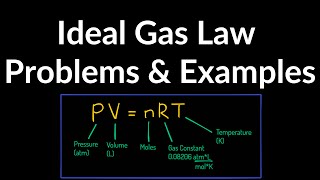
Textbook QuestionModern vacuum pumps make it easy to attain pressures of the order of 10^-13 atm in the laboratory. Consider a volume of air and treat the air as an ideal gas. (a) At a pressure of 9.00 * 10^-14 atm and an ordinary temperature of 300.0 K, how many molecules are present in a volume of 1.00 cm^3?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
260
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos